Real World Evidence Demonstration Project
Expanding labeling for a cryoablation probe to adolescent patients
The UCSF-Stanford Pediatric Device Consortium is working with government and industry partners to expand access to pediatric devices by using real world evidence (RWE) in new and innovative ways. RWE pertains to the potential risks and benefits of a device or drug identified via data derived outside of traditional clinical trial settings.
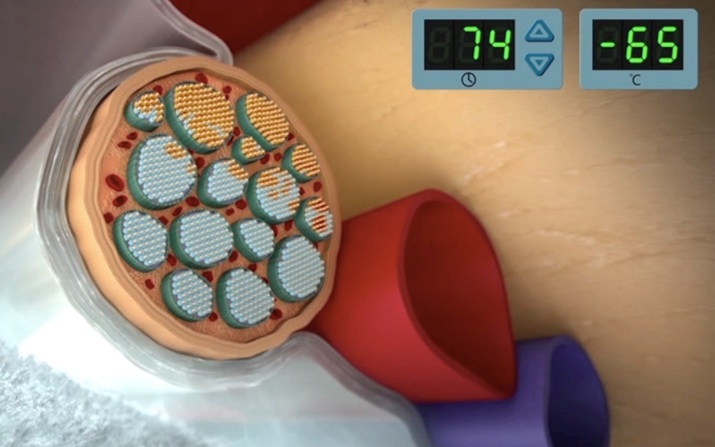
In our demonstration project, the PDC is collaborating with AtriCure to expand labeling for its cryoICE® CRYO2 cryoablation probe to adolescent patients. By temporarily ablating peripheral nerves in the chest, Cryo Nerve Block Therapy (cryoNB) offers the possibility of substantially reducing the postoperative pain associated with Nuss surgery for pectus excavatum (“sunken chest”) repair.
Using innovative Electronic Health Record (EHR) data mining methods, the PDC is assembling a broader picture of cryoNB’s safety and effectiveness in adolescent Nuss patients to expand the clinical evidence base beyond the traditional RCT. In the next phase of the project, the PDC will be investigating data collected from mobile health applications and connected sensors.
Project Partners:
UCSF-Stanford Center of Excellence in Regulatory Science and Innovation (CERSI)
UCSF Benioff Children’s Hospital Division of Pediatric Surgery